Fistula arterio-venoasa pulmonara / Tratament legatura anormala artera si vena pulmonara
| Anestezie | locală |
| Durată procedură | 30 min |
| Durată spitalizare | 24 h |
Cuprins
- Generalități despre fistula arterio-venoasă pulmonară
- Situatii
- Cat de frecvente sunt MAVP?
- Alte asocieri non-cardiace:
- Complicatiile asociate MAVP includ:
- Diagnosticul MAVP
- Alte teste
- Proceduri efectuate pentru inchiderea MAVP:
- Cum te pregătești pentru intervenție?
- Cât durează internarea?
- Riscuri
- Ce se întâmplă în timpul procedurii
- Dispozitive Amplatzer
- Ce se întâmplă după procedură?
- Ce trebuie să faci după ce ieși din spital?
Generalități despre fistula arterio-venoasă pulmonară
Malformațiile arterio-venoase pulmonare (numite și fistule arterio-venoase pulmonare – MAVP) se referă la comunicări anormale între arterele și venele pulmonare.
Majoritatea pacienților cu MAVP asociază boala numită teleangiectazia ereditară hemoragică (HHT; este o afecțiune cu transmitere genetică).
Unele MAVP sunt dobândite, asa cum se întâmplă în cazul pacienților cu boli hepatice, mai ales ciroza hepatică, pacienți cu boli cardiace congenitale. De asemenea, MAVP pot să apară în infecții cronice (de ex: schistosomiază, actinomicoză, tuberculoză), în cancer tiroidian metastatic, stenoză mitrală, bronsiectazii.
HHT este o boală cu transmitere genetică care se caracterizează prin prezența malformațiilor vasculare la nivelul diferitelor organe, cel mai frecvent la nivelul pielii, nazofaringelui, tractului digestiv, plămânilor și creierului. De obicei, diagnosticul se bazează pe o asociere de trei situații:
Situatii
1. teleangiecatzii cutanate
2. epistaxis recurent (sângerare nazală repetată)
3. istoric familial al bolii.
Aproximativ 70% din pacienții cu MAVP prezintă HHT. Invers, cca 15-30% din pacienții diagnosticați cu HHT au si MAVP.
Cca 53-70% dintre MAVP sunt localizate în lobii inferiori pulmonari.
Majoritatea au boala unilaterală, unii au leziuni multiple, iar dintre aceștia, jumătate au boala localizată în ambii plămâni. MAVP pot fi microscopice (teleangiectazii), dar, tipic, ating dimensiuni de 1-5cm. Ocazional, pot atinge mărimi de 10cm.
10% dintre pacienți, prezintă asociere dintre leziuni microscopice difuze și leziuni de dimensiuni mari, vizibile radiologic.
Aceste comunicări anormale se deschid fie în atriul stâng (majoritatea), fie la nivelul venei cave inferioare și se pot prezenta sub formă de sac unic, ca o rețea (aspect plexiform), ca niște canale dilatate sau ca niște legături directe, foarte sinuoase, între artere și vene pulmonare.
MAVP se clasifică de asemenea, în funcție de arhitectură, în simple (majoritatea) sau complexe. Cele simple primesc sânge dintr-un singur ram arterial și drenează într-un singur segment venos. Acestea se găsesc mai ales în partea inferioară a plămânilor. Cele complexe, cca 21%, prezintă cel puțin 2 segmente arteriale/venoase care alimentează sau în care drenează; ele se găsesc mai ales în porțiunea medie pulmonară.
Evoluția naturală a MAVP nu este elucidată. Un studiu mic (care a inclus 16 pacienți) a arătat că rata lor de creștere pare să fie mică, cu o creștere de cca 5-10 mm la fiecare 5-15 ani. De asemenea, regresia aproape în totalitate a anomaliei a fost observată la un pacient.
Cat de frecvente sunt MAVP?
Așa cum am precizat deja, MAVP se asociază în cca 70% din cazuri cu HHT și pacienții cu HHT prezintă în cca 15-35% din cazuri și MAVP. Deși HHT este o boală genetică, nu întotdeauna există și istoric familial al bolii. În cazul HHT anomaliile vasculare sunt prezente încă de la naștere, dar manifestările clinice apar în mod normal în cursul vieții de adult, pe măsură ce vasele au fost supuse unor presiuni, pe perioade mai mari de timp.
Mortalitatea asociată MAVP se datorează rupturii, abceselor cerebrale și infarctului cerebral, secundar emboliei paradoxale. Prin comparație, intervențiile de închidere a MAVP se asociază cu un risc mai scăzut. Riscul este cu atât mai mare cu cât leziunile sunt mai difuze sau bilateale.
De asemenea, MAVP sunt de două ori mai frecvente la femei (deși la naștere există o ușoară predominață a sexului masculin), cca 10% sunt diagnosticate în copilărie, dar frecvența bolii este mai mare la vârsta adultă (după 40-50 de ani).
SIMPTOME
Simptomele se instalează lent, pe măsură ce MAV cresc în dimensiuni.
Pe parcursul anilor poate să apară dispnee de efort (respirație dificilă la efort). În cazuri severe, poate să apară dispnee în poziția în picioare (platipnee). De asemenea, poate să apară cianoză (colorație albastră a tegumentelor și mucoaselor).
Unii pacienți acuză hemoptizii (sângerare din căile respiratorii) – pot fi severe.
Mai rar, pacienții afirmă angina, tuse, migrena, tinitus („tiuit” in urechi), amețeală, dizartrie (vorbire dificilă), sincop (pierderea stării de conștiență), vertij, diplopie (tulburare de vedere – vedere dublă).
Întrucât 70% din MAVP sunt întâlnite la pacienții cu HHT, aceștia prezintă frecvent leziuni la nivelul pielii și mucoaselor (teleangiectazii) – mai frecvent pe față, gură, piept și membre superioare.
Alte asocieri non-cardiace:
1. Cel mai frecvent este afectat sistemul nervos central (30% din cazuri):
• accident vascular cerebral – 18% din pacienții cu afectare SNC
• accident ischemic tranzitor – 37%
• abces cerebral – 9%
• migrena – 43%
• convulsii – 8%.
Explicația acestora vine din embolizarea paradoxală ( inclusiv embolizare de material infecțios ce duce la producerea abceselor cerebrale). Cel mai adesea, în acest caz, arterele care alimentează anomalia vasculară au dimensiuni peste 3mm.
2. Hemoptizii și hemotorax (acumulare de sânge în pleură – învelișul plămânilor), secundar ruperii vaselor care formează MAVP.
MAVP idiopatice:
MAVP idiopatice sunt unice, cel mai frecvent. În plus, majoritatea au risc mai mic de progresie, semnele clinice sunt mai sărace, dar au aceeași indicație de tratament.
MAVP dobândite după intervenții chirurgicale pentru boli cardiace congenitale:
Pot să apară după anumite procedee chirurgicale de corecție a cardiopatiilor congenitale cianogene. În scop diagnostic se utilizează teste de tipul ecografiei cardiace cu contrast și studii utilizând radionuclizi, iar tratamentul lor constă în embolizare.
Complicatiile asociate MAVP includ:
• convulsii
• migrena
• accident ischemic tranzitor sau accident vascular cerebral
• abces cerebral
• hipoxemie, ortodeoxie
• hemotorax
• hemoptizii (incluziv amenintatoare de viata)
• hipertensiune pulmonară
• insuficiență cardiacă
• policitemie
• anemie
• endocardita infecțioasă.
Diagnosticul MAVP
MAVP trebuie considerate la orice individ care se prezintă cu oricare dintre:
1. unul sau mai mulți noduli pulmonari evidențiați la radiografia pulmonară, cu aspect sugestiv de MAVP
2. teleangiectazii muco-cutanate
3. simptome precum: dispnee, hemoptizie, hipoxemie, policitemie, cianoză, embolie cerebbrala, abces cerebral.
Unele teste de laborator sunt modificate (hipoxemie cronică, cu hemoglobină și hematocrit crescute; dar, anemia poate fi prezentă, mai ales la pacienții cu HHT, din cauza sângerărilor posibile).
Puls-oximetria: poate fi folosită ca test screening pentru MAVP. Se realizează atât în poziție culcat, cât și în ortostatism (în picioare) – în acest ultim caz, valorile scăzând mult.
Imagistica are un rol important:
• radiografia toracică
• ecografia cardiaca: principalul rol este de a exclude alte cauze de sunt dreapta-stânga.
• ecografie cardiacă cu contrast
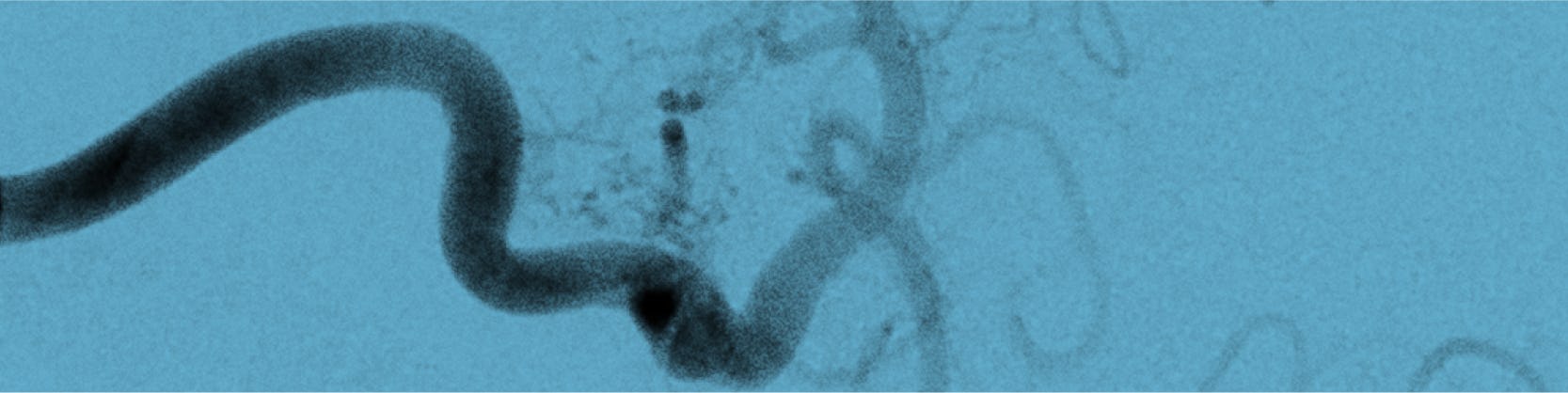
• angiografie pulmonară: rămâne criteriul standard pentru diagnostic și este absolut necesară în caz de emboloterapie. Se efectuează la nivelul tuturor lobilor pulmonari pentru a evidenția toate MAVP.
• cateterism cardiac
• teste de perfuzie pulmonară cu radionuclizi
• imagistică prin rezonanță magnetică
• CT cu contrast.
Alte teste
Teste pentru evaluarea funcției pulmonare: oxigenarea este frecvent afectată la acești pacienți (de obicei saturație <90%). De obicei ei au saturație scăzută, dar cu valori spirometrice normale.
Toleranța la efort: scăzută în general. Pe măsura ce crește gradul de efort, saturația în oxigen scade.
INDICAȚII
În cazul MAVP, tratamentul medicamentos nu reprezintă o opțiune, exceptând situațiile în care este necesară profilaxia antibiotică, înainte de proceduri stomatologice și chirurgicale, cu scopul de a preveni embolizarea infecțioasă cu abces cerebral secundar.
Nu toate MAVP necesită tratament imediat. Alegerea momentului operator trebuie să țină cont de raportul risc-beneficiu ( riscul complicațiilor MAVP vs. riscul asociat procedurii).
Indicațiile de tratament nu au fost clar stabilite, dar opinia experților este de a se interveni dacă:
există una sau mai multe MAVP cu artera implicată având diametrul între 2 si 3 mm (cuantificat computer tomograf), indiferent de simptome – în acest caz se practică angiografie pulmonară și dacă vasul arterial implicat are peste 3 mm atunci se efectuează embolizare, iar dacă este <3mm, embolizarea se va practica doar dacă este tehnic posibilă (întrucât evoluția naturală implică mărirea progresivă și apariția simptomatologiei în timp)
MAVP sunt simptomatice, indiferent de mărimea arterei implicate
MAVP asimptomatice, cu artera sub 2 mm ar trebui urmărite periodic prin computer tomograf, la 3-5 ani, cu posibilitate de tratament dacă se măresc sau pacienții devin simptomatici.
Astfel, terapia MAVP implică fie tratamentul intervențional (embolizare), fie corecția chirurgicală.
Proceduri efectuate pentru inchiderea MAVP:
1. embolizarea transcateter- este o procedură minim invazivă
2. corecție chirurgicală – reprezintă o opțiune doar în cazul pacienților alergici la substanța de contrast sau care au MAVP care tehnic nu pot fi corectate prin embolizare. în plus, nu este întotdeauna curativă pe termen lung – până la 12% din pacienți prezintă recurența fistulei; s-au raportat și mărirea MAVP care nu a fost diagnosticată anterior, dar și AVC. Presupune ligatura comunicării, excizie locală, lobectomie sau pneumectomei.
Cum te pregătești pentru intervenție?
 1. Vei efectua analize de sânge
1. Vei efectua analize de sânge 2. Nu vei mânca și nu vei bea apă
2. Nu vei mânca și nu vei bea apă
Cât durează internarea?
Tratamentul intervențional prezintă avantajul unei internări de scurtă durată și a unei perioade scurte de convalescență. Perioada de internare variază între 24 și 48 de ore, timp în care vor fi efectuate analize, investigații și procedura de diagnosticare și tratament. Te poți întoarce la viața ta, în numai câteva zile.
Riscuri
Complicațiile sunt rare și sunt evitate printr-o pregătire și supraveghere corespunzătoare. Complicațiile posibile includ:
• reacții alergice la administrarea substanței de contrast, inclusiv insuficiență renală
• reacții la compușii anestezici
• fistule arteriovenoase la locul puncției
• sângerări la locul puncției
• febră
• cefalee (durere de cap), migrena
• infecții
• embolie gazoasă
• aritmii cardiace
• foarte rară – perforație cardiacă și tamponada cardiacă
• hemoragii, afectare vasculară după dilatarea balonului
• greața, vărsături
• durere pleuritică- cea mai comună complicație, care răspunde bine la analgezie ( nonsteroidiana, deși, în anumite situații se poate recurge la administrare de prednison)
• infarct pulmonar
• tromboze arteriale sau venoase
• migrarea dispozitivului
• hipertensiune pulmonară - fie nouă, fie agravarea celei preexistente
• recanalizare
• accident vascular cerebral sau accident ischemic tranzitor
Ce se întâmplă în timpul procedurii
Embolizarea este o formă de tratament care presupune ocluzia arterei care alimentează malformația. Materialele emboligene utilizate sunt: dispozitive de tip „coil”, alcool polivinil, dar și dispozitive de tip Amplatzer (mai nou utilizate).
Inițial se practică angiografie de diagnostic, pentru o mai bună caracterizare a MAVP, pentru diagnosticul tuturor MAVP – se pot emboliza în aceeași ședință mai multe MAVP, dar, de asemenea, se pot efectua embolizări succesive, la intervale de 1-2 săptămâni.
MAVP difuze reprezintă o provocare: închiderea lor are ca și țintă reducerea complicațiilor asociate, chiar dacă în ceea ce privește hipoxemia nu sunt rezultate remarcabile. În plus, se obține totuși o îmbunătățire a simptomatologiei.
Cel mai frecvent utilizate sunt dispozitive de tip coil metalice (în acest caz, ele trebuie fixate cât mai aproape de sacul fistulei și trebuie să fie mai mari decât grosimea arterei implicate, de obicei cu 2 mm) sau dispozitive de tip balon-detasabil (prezintă avantajul de a putea fi dezinflate și repoziționate).
Dispozitive Amplatzer
Așa cum am precizat, se mai pot utiliza și dispozitive Amplatzer – relativ recent. Avantajul acestora vine din faptul că realizează mai rapid ocluzia MAVP, scade durata procedurii, pot fi închise mai multe MAVP în cadrul aceleiași ședinte, pot fi abordate MAVP cu dimensiuni mai reduse, se pare că există un risc mai mic de embolie paradoxală în cursul procedurii cu o rată mai mică de reperfuzie tardivă a MAVP.
Procedura se efectuează în laboratorul de angiografie și de obicei se practică sub anestezie generală. După asepsia locală, medicul intervenționist va efectua mici incizii la nivelul canalului inghinal ( la rădăcina coapsei) pentru a vizualiza vena femurală. La nivelul acestei mici incizii, sub ecran radiologic, se va introduce o teacă, ulterior pe teacă se introduc catetere până la nivelul ventriculului drept. Cateterul va fi poziționat cât mai aproape de gâtul MAVP.
Pe cateter se avansează dispozitivul utilizat – de tip coil, și eliberat așa încât să obstrueze MAVP. Se repetă angiografia și, la nevoie, alte dispozitive pot fi utilizate (chiar până la 10).
În cazul utilizării balonului detașabil, acesta, odată poziționat în locul dorit, va fi umflat cu substanță de contrast radioopacă, se repetă angiografia pentru a evidenția închiderea fistulei și, ulterior, balonul va fi detașat de cateter. Avantajul, asa cum am precizat mai sus, constă în faptul că, la nevoie, balonul poate fi dezumflat și repoziționat pentru a obține rezultate optime. Au fost raportate situații de dezumflare a balonului postprocedural, pentru aceasta se pot utiliza alte tipuri de substanțe – izotonice cu sângele.
În cazul MAVP cu dimensiuni între 7-10 mm se pot utiliza ambele tipuri de device-uri.
Ce se întâmplă după procedură?
 1. Vei bea cel puțin 2 litri de apă, pentru a elimina substanța de contrast.
1. Vei bea cel puțin 2 litri de apă, pentru a elimina substanța de contrast. 2. Ti se va monitoriza pulsul, tensiunea și temperatura
2. Ti se va monitoriza pulsul, tensiunea și temperatura 3. Vei fi externat
3. Vei fi externat
Ce trebuie să faci după ce ieși din spital?
Dacă ai trecut printr-o astfel de procedură, este important să revii la control așa cum ți-a recomandat medicul tău și să respecți tratamentul indicat de acesta. Îți poți relua activitatea imediat. Nu neglija mișcarea fizică moderată, respectă un regim de viață echilibrat și fă-ți regulat analizele de sânge.
Locații MONZA ARES în care se efectuează Fistula arterio-venoasa pulmonara / Tratament legatura anormala artera si vena pulmonara
Procedurile Noastre
- Investigații cardiologie
- Electrocardiograma - ECG / EKG
- Consult Cardiologic
- Ecocardiografie / Ecografie cardiacă
- Ecografie doppler carotidiana
- Ecografia doppler de artere / vene
- Holter EKG - Monitorizarea ritmului inimii 24H
- Holter TA - Monitorizare tensiune arteriala
- Ecografia cu dobutamină / Ecografia de stres
- Test de efort / Ergometrie
- Ecocardiografia transesofagiană
- Programare Stimulator Cardiac
- Evaluare si control cardiovascular complet post COVID-19
- Ecografia cu bule / cu substanță de contrast
- Ecocardiografia speckle tracking
- Consultație diabetologie
- Indice Gleznă/Braț
- Consultație cardiologie pediatrică
- Programul de readaptare cardiacă/ recuperare cardiovasculară post COVID-19
- Investigații neurologie
- Bolile valvelor și ale vaselor inimii
- Coronarografie - Angiografie coronariana / Diagnostic angina pectorala
- Angioplastie coronariană cu stenturi / Tratament angină pectorală ischemie cardiacă
- Tratament stenoză aortică – TAVI – Implantare valvă aortică
- Tratament insuficiență mitrală - MitraClip
- Valvuloplastie mitrala cu balon / Tratament stenoză mitrală hipertensiune pulmonara
- Revascularizarea ocluziilor coronariene cronice totale (CTO)
- Rotablație coronariană - Tratament angină pectorală (artere calcifiate)
- Procedura JetStream – sistem de aterectomie pentru dezobstrucția arterelor sever calcifiate
- Terapia Shockwave / Tratament boala cardiacă ischemică – artere calcifiate
- Implantare de stent Flow Reducer / Tratament angina pectorala rezistenta la medicație
- Chirurgia valvei mitrale / Operatie chirurgicala insuficienta mitrala
- Bypass aorto-femural și femuro-popliteal / Operatie pentru ateroscleroză
- Bypass aorto-coronarian / Operatie chirurgicala pentru angină pectorală
- Aritmii cardiace
- Ablatie fibrilatie atriala | Tratament fibrilatie atriala
- Ablatie flutter atrial / Tratament flutter atrial
- Ablatie tahicardie jonctionala / Tratament tahicardie joncțională
- Implantare stimulator cardiac / Tratament dereglări de ritm cardiac
- Implantare Defibrilator cardiac / Corectarea prin șoc electric a ritmului inimii
- Sindrom WPW | Wolff-Parkinson-White
- Procedura Watchman pentru fibrilatia atriala / Tratament preventiv AVC in fibrilatia atriala
- Tahicardie | AVNRT | Tahicardia prin reintrare in nodul atrio-ventricular
- Monitorizarea electrocardiografica pe termen lung | LOOP RECORDER
- Studiu electrofiziologic / Identificarea zonelor de aritmie cardiaca
- Cardioversie electrica – restabilirea ritmului normal al inimii
- Bradicardie | Tratament aritmie cardiaca lenta
- Ablatia prin radiofrecventa | Crioablatia / Tratament tahiaritmii cardiace
- Defecte (malformații) cardiace din naștere
- Bolile vaselor de sânge
- Embolizare hemoroizi / Tratament minim invaziv hemoroizi
- Angiografia generală / Diagnostic artere blocate
- Anevrismul de aorta / Tratament aorta dilatata
- Implantare de stent graft - Disecție de aortă de tip B
- Angiografia de artere mezenterice - angioplastie cu balon și stent / Diagnostic si tratament ingustare vase de sange sistem digestiv
- Angiografia de artere periferice - angioplastie cu balon si stent / Diagnostic si tratament ingustare vase de sange maini si picioare
- Angioplastia de artere renale / Tratament ingustare vase de sange rinichi – reducere hipertensiune arteriala
- Angioplastie artere subclavii / Tratament ingustare vase de sange gat
- Implantare de filtru de vena cava inferioara / Preventie embolie pulmonara
- Denervarea arterelor renale / Tratament minim invaziv hipertensiune arterială rezistenta la tratament
- Angioplastie venoasa cu balon si stent
- Fibroid Center
- Embolizare fibrom uterin / Tratament minim invaziv fibrom uterin
- Consultatia ginecologica
- Ecografia transvaginala / endovaginala
- Test HPV (Papilloma Virus Uman)
- Test Babes-Papanicolau
- Ginecologie - Trichomonas Vaginalis
- Ginecologie - Candidoza vaginala
- Colposcopie
- Electrorezectia / Prelevare țesut col uterin
- Ecografia de morfologie fetala - Trimestrul I
- Ecografia de morfologie fetala - Trimestrul II
- Ecografia de morfologie fetala - Trimestrul III
- Boli oncologice si tratamente tumorale
- Bolile valvelor și ale vaselor inimii la copii
- Embolizare hemangiom / Tratament minim invaziv tumori benigne
- Fistula arterio-venoasa coronariana / Tratament legatura anormala artera si vena
- Fistula arterio-venoasa pulmonara / Tratament legatura anormala artera si vena pulmonara
- Stenoza pulmonara periferica / Tratament ingustare artera pulmonara
- Valvuloplastie pulmonara cu balon / Tratament ingustare valva pulmonara
- Boli ale aparatului genital masculin
- Boli cerebrale și ale coloanei vertebrale
- Anevrismul cerebral / Tratament durere de cap, paralizii preventie AVC
- Angioplastie carotidiana / Tratament boala carotidiana, atac ischemic vascular, AVC
- Discectomie cervicala cu DiscoGel / Tratament hernie de disc cervicala
- Malformatii arterio-venoase cerebrale / Tratament hemoragii cerebrale
- Nucleoplastie / Tratament hernie de disc
- Vertebroplastie / Tratament dureri coloana vertebrala fracturi de compresie
- Discectomie cu alcool
- Infiltrații transforaminale
- Boli ale articulațiilor

