Malformatii arterio-venoase cerebrale / Tratament hemoragii cerebrale
| Anestezie | generală |
| Durată procedură | 30-60 min |
| Durată spitalizare | 24h |
Cuprins
- Generalități despre malformațiile arterio-venoase cerebrale
- Care sunt cauzele MAV?
- Simptome
- Indicații
- Cum te pregătești pentru tratamentul intervențional?
- Cât durează internarea?
- Riscuri
- Hemostaza
- Ce se întâmplă după procedură?
- Ce trebuie să faci după ce ieși din spital?
Generalități despre malformațiile arterio-venoase cerebrale
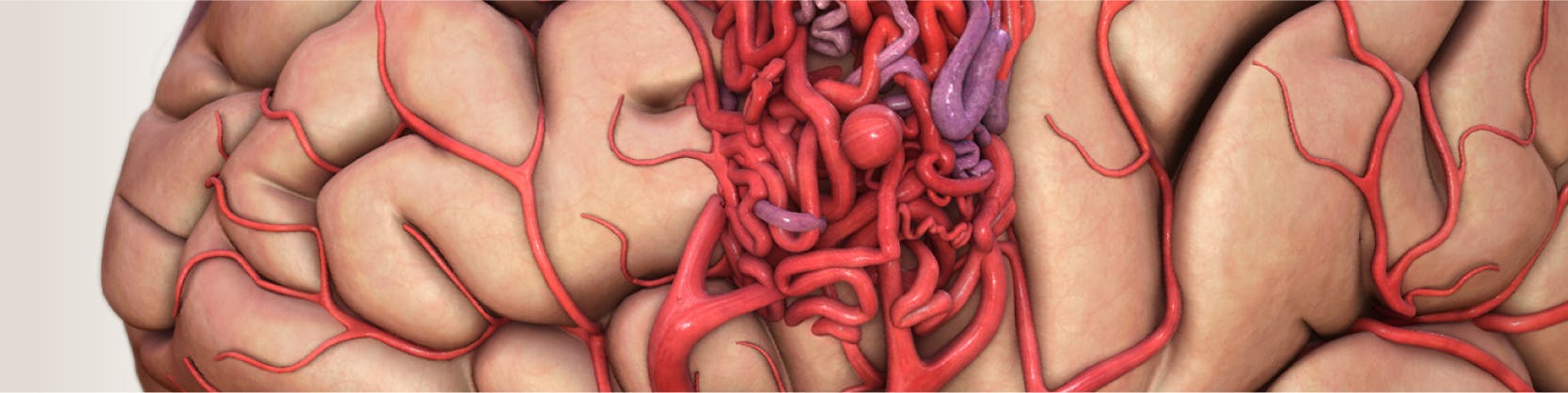
MAV reprezintă o conexiune anormală și fragilă (ca un nod, ghem) între artere și vene. Această anormalitate se asociază cu un risc crescut de hemoragie. Pot fi localizate oriunde în corp, dar cele localizate la nivel cerebral comportă un risc crescut datorită posibilității de sângerare cu complicații grave secundare. Cele localizate în vasele învelișurilor creierului se numesc malformații arterio-venoase durale.
Care sunt cauzele MAV?
Cauzele MAV sunt necunoscute. Există diferite ipoteze, unele dintre acestea sugerând că se datorează unei anomalii de dezvoltare în viața intrauterine (în timpul sarcinii când se formează fătul), deci ele există încă de la naștere. Majoriatea nu sunt ereditare și au o frecvență egală pe sexe.
De obicei sunt diagnosticate la 20-40 de ani, în urma complicațiilor de tip sângerare (risc de 4% pe an – asta înseamnă că din 100 de personae care au această anomalie 4 vor sângera din malformație în fiecare an) sau convulsii.
Simptome
Cel mai adesea MAV sunt semnalate de:
- convulsii ( existența lor poate fi iritantă la nivel cerebral generând semnale electrice anormale)
- cefalee (durere de cap) intense, de tip migrenos
- simptome de tip stroke-like (adică simtomatologie ca în cazul accidentului vascular cerebral) din cauza faptului că țesuturile din jur nu primesc în cantitate suficientă oxigen și substanțe nutritive pentru buna funcționare. Aceste simptome depind de localizarea malformației și pot include: slăbiciune și paralizie, tulburări vizuale, auditive, de echilibru, de memorie sau de personalitate.
În caz de hemoragie manifestările includ cefalee severă, bruscă, greață, vărsătură, ceafă rigidă, tulburări neurologice (ca cele menționate anterior), pierderea stării de conștiență, deces.
Nu trebuie neglijat faptul ca pot fi asimptomatice (dar, atenție, riscul de sângerare este existent și în această situație!).
De menționat ca în cazul unei eventuale sarcini, riscul de sângerare este mai mare, mai ales după primul trimestru de sarcină, de aceea, deși MAV nu contraindicp sarcina, este prudent ca aceasta să fie amânată până ce MAV va fi tratată corespunzător.
Diagnosticul acestei anomalii poate fi pus în urma unor investigații imagistice: angio CT, angio RMN, angiograme cerebrale.
Indicații
MAV se asociază cu un risc de sângerare de 4% pe an. Hemoragia poate duce la complicații neurologice severe ( accident vascular cerebral, paralizii permanente, deces) de aceea se recomandă tratamentul acestor anomalii vasculare. Acesta se poate realiza chirurgical ( tehnici de microneurochirurgie) sau intervențional. Tratamentul minim invaziv presupune injectarea unor substanțe asemănătoare unui lipici care închide total sau parțial malformația. O altă metodă ar înseamna tehnicile de radio-chirurgie (presupun administrarea locală de radiații).
Decizia de tratament ia iîn considerare localizarea anomaliei, precum și consecințele posibile.
Alegerea tipului de intervenție se face ținând cont de riscul cel mai mic asociat procedurii pentru care se optează și de posibilitatea cea mai mare de închidere a anomaliei.
În ceea ce privește tratamentul intervențional, acesta:
• se asociază cu o rată de vindecare într-o singură sedință de 20%,
• pot fi necesare mai multe sesiuni pentru rezultate optime, se poate repeta la scurt timp fără consecințe severe
• are risc mai redus decât intervenția chirurgicală, nu necesită incizie chirurgicală astfel spitalizare este mai redusă și recuperarea mai rapidă
• poate reduce dimensiunile unei MAV astfel încât aceasta să fie apoi abordată fie chirurgical fie radiologic, cu un profil de siguranță mai mare
• închiderea parțială se asociază în continuare cu riscul de hemoragie, desi acesta va fi mai scăzut
• se poate face în scop paliativ (când nu se așteaptă să se trateze, ci doar să amelioreze simptomele și calitatea vieții).
(ex: la pacienți cu afectare neurologică secundar edemului cerebral – acumulare de apă la nivel cerebral sau datorată sindromului de furt – când o zonă de creier este deprivată de sânge, acesta fiind redirecționat către alta zonă).
• depinde de anatomia sistemului arterial (existența unor vase tortuoase (cudate) poate împiedică traiectul cateterelor).
Cum te pregătești pentru tratamentul intervențional?
 1. Vei efectua un set complet de analize de sânge
1. Vei efectua un set complet de analize de sânge 2. Anumite medicamente vor fi oprite
2. Anumite medicamente vor fi oprite 3. Nu vei mânca și nu vei bea apă
3. Nu vei mânca și nu vei bea apă
Cât durează internarea?
Tratamentul intervențional prezintă avantajul unei internări de scurtă durată și a unei perioade scurte de convalescență. Perioada de internare variază între 24 și 48 de ore, timp în care vor fi efectuate analize, investigații și procedura de embolizare. Te poți întoarce la viața ta, în numai câteva zile.
Riscuri
- reacții alergice la substanțele administrate
- reacții la anestezice
- fistule arteriovenoase la nivelul puncției vasculare
- mici sângerări la nivelul puncției vasculare
- febra
- cefalee, migrenă
- infecție
- embolie gazoasă
- lezarea peretului aortic, al arterei femurale sau ai altor artere
- risc scăzut de deces și accident vascular cerebral
- inconstant, cu durată de la câteva zile la câteva săptămâni, pacientul poate descrie cefalee (dacă este persistă, mediul curant poate prescrie medicamente antialgice)
- de asemenea, pot fi diferite manifestări neurologice(de ex: slăbiciune și paralizii membre, tulburări de vedere sau de vorbire)
Tratamentul intervențional – numit în acest caz embolizare – este o tehnică minim invazivă care presupune injectarea unui material ( ca un lipici/ altă substanță lichidă adezivă non-reactivă) la nivelul MAV. Odată injectată, substanța se întărește rapid, astfel încât să blocheze total sau parțial sângele la acest nivel. După anestezie se efectuează o mică incizie la nivel inghinal pentru a vizualiza artera femurală. Aici se va introduce o teacă (un tub de plastic care permite manipularea echipamentului necesar intervenției). Ulterior se administrează heparina care previne formarea cheagurilor de sânge în timpul intervenției. Apoi se introduce pe teacă un cateter până la locul leziunii. Se practică angiograme seriate cu ajutorul cărora se verifică constant poziția cateterului. În plus, se verifică încă o dată dacă malformația vasculară se pretează la tratament intervențional. În momentul În care se ajunge la nivelul anomaliei se injectează substanța. Odată eliberată în interiorul malformației, se întărește și oprește astfel alimentarea cu sânge. La finalul injectării se verifică statusul obstrucției prin angiografie. Uneori, mai ales în cazul MAV voluminoase, pot fi necesare sedințe repetate de embolizare, cu obstrucții parțiale seriate, până la închidere totală.
În momentul în care se consideră încheiată embolizarea ( fie s-a obținut obstrucția totală, fie se consideră că în cadrul acelei sedințe nu se mai poate tenta nimic) se retrag cateterele, teaca putând a fi lăsată pe loc pentru câteva ore.
Hemostaza
După terminarea procedurii se scot cateterele și tecile arteriale, iar la locul de puncție se realizează compresiunea arterei folosite ca abord. Pentru a se obține compresia, se pune un pansament compresiv ce trebuie păstrat timp de 24 de ore pentru a se evita sângerările.
În ambele cazuri de abord, pacientului i se va indica să păstreze repaus la pat. În anumite situații, există indicația de a monta un sistem hemostatic la nivelul puncției femurale. Acesta se montează în sala de angiografie, imediat după terminarea intervenției. AngioSeal este mic dop de colagen și se introduce în interiorul arterei femurale. Acesta are ca efect obținerea hemostazei în doar două ore. Astfel, pacientul își poate mișca piciorul în voie și se poate ridica din pat.
Ce se întâmplă după procedură?
 1. Vei bea cel puțin 2 litri de apă, pentru a elimina substanța de contrast.
1. Vei bea cel puțin 2 litri de apă, pentru a elimina substanța de contrast. 2. Vei face o electrocardiogramă de control
2. Vei face o electrocardiogramă de control 3. Pansamentul de la locul puncției se scoate la 12-24 de ore.
3. Pansamentul de la locul puncției se scoate la 12-24 de ore. 4. Vei face analize de sânge.
4. Vei face analize de sânge.
Ce trebuie să faci după ce ieși din spital?
Dacă ai trecut printr-o astfel de procedură, este important să revii la control așa cum ți-a recomandat medicul cardiolog și să respecți tratamentul indicat de acesta. Îți poți relua activitatea imediat. Nu neglija mișcarea fizică moderată, respectă un regim de viață echilibrat și fă-ți regulat analizele de sânge.
MAV se asociază cu un risc de sângerare care poate avea consecinț severe (inclusive decesul), de aceea este importantă o atitudine terapeutică optimă, înainte de dezvoltarea complicațiilor!!
În acest sens, metoda intervențională de tratament permite o rezolvare eficientă cu perioadă de recuperare scurtă și spitalizare redusă.
Locații MONZA ARES în care se efectuează Malformatii arterio-venoase cerebrale / Tratament hemoragii cerebrale
Procedurile Noastre
- Investigații cardiologie
- Electrocardiograma - ECG / EKG
- Consult Cardiologic
- Ecocardiografie / Ecografie cardiacă
- Ecografie doppler carotidiana
- Ecografia doppler de artere / vene
- Holter EKG - Monitorizarea ritmului inimii 24H
- Holter TA - Monitorizare tensiune arteriala
- Ecografia cu dobutamină / Ecografia de stres
- Test de efort / Ergometrie
- Ecocardiografia transesofagiană
- Programare Stimulator Cardiac
- Evaluare si control cardiovascular complet post COVID-19
- Ecografia cu bule / cu substanță de contrast
- Ecocardiografia speckle tracking
- Consultație diabetologie
- Indice Gleznă/Braț
- Consultație cardiologie pediatrică
- Programul de readaptare cardiacă/ recuperare cardiovasculară post COVID-19
- Investigații neurologie
- Bolile valvelor și ale vaselor inimii
- Coronarografie - Angiografie coronariana / Diagnostic angina pectorala
- Angioplastie coronariană cu stenturi / Tratament angină pectorală ischemie cardiacă
- Tratament stenoză aortică – TAVI – Implantare valvă aortică
- Tratament insuficiență mitrală - MitraClip
- Valvuloplastie mitrala cu balon / Tratament stenoză mitrală hipertensiune pulmonara
- Revascularizarea ocluziilor coronariene cronice totale (CTO)
- Rotablație coronariană - Tratament angină pectorală (artere calcifiate)
- Procedura JetStream – sistem de aterectomie pentru dezobstrucția arterelor sever calcifiate
- Terapia Shockwave / Tratament boala cardiacă ischemică – artere calcifiate
- Implantare de stent Flow Reducer / Tratament angina pectorala rezistenta la medicație
- Chirurgia valvei mitrale / Operatie chirurgicala insuficienta mitrala
- Bypass aorto-femural și femuro-popliteal / Operatie pentru ateroscleroză
- Bypass aorto-coronarian / Operatie chirurgicala pentru angină pectorală
- Aritmii cardiace
- Ablatie fibrilatie atriala | Tratament fibrilatie atriala
- Ablatie flutter atrial / Tratament flutter atrial
- Ablatie tahicardie jonctionala / Tratament tahicardie joncțională
- Implantare stimulator cardiac / Tratament dereglări de ritm cardiac
- Implantare Defibrilator cardiac / Corectarea prin șoc electric a ritmului inimii
- Sindrom WPW | Wolff-Parkinson-White
- Procedura Watchman pentru fibrilatia atriala / Tratament preventiv AVC in fibrilatia atriala
- Tahicardie | AVNRT | Tahicardia prin reintrare in nodul atrio-ventricular
- Monitorizarea electrocardiografica pe termen lung | LOOP RECORDER
- Studiu electrofiziologic / Identificarea zonelor de aritmie cardiaca
- Cardioversie electrica – restabilirea ritmului normal al inimii
- Bradicardie | Tratament aritmie cardiaca lenta
- Ablatia prin radiofrecventa | Crioablatia / Tratament tahiaritmii cardiace
- Defecte (malformații) cardiace din naștere
- Bolile vaselor de sânge
- Embolizare hemoroizi / Tratament minim invaziv hemoroizi
- Angiografia generală / Diagnostic artere blocate
- Anevrismul de aorta / Tratament aorta dilatata
- Implantare de stent graft - Disecție de aortă de tip B
- Angiografia de artere mezenterice - angioplastie cu balon și stent / Diagnostic si tratament ingustare vase de sange sistem digestiv
- Angiografia de artere periferice - angioplastie cu balon si stent / Diagnostic si tratament ingustare vase de sange maini si picioare
- Angioplastia de artere renale / Tratament ingustare vase de sange rinichi – reducere hipertensiune arteriala
- Angioplastie artere subclavii / Tratament ingustare vase de sange gat
- Implantare de filtru de vena cava inferioara / Preventie embolie pulmonara
- Denervarea arterelor renale / Tratament minim invaziv hipertensiune arterială rezistenta la tratament
- Angioplastie venoasa cu balon si stent
- Fibroid Center
- Embolizare fibrom uterin / Tratament minim invaziv fibrom uterin
- Consultatia ginecologica
- Ecografia transvaginala / endovaginala
- Test HPV (Papilloma Virus Uman)
- Test Babes-Papanicolau
- Ginecologie - Trichomonas Vaginalis
- Ginecologie - Candidoza vaginala
- Colposcopie
- Electrorezectia / Prelevare țesut col uterin
- Ecografia de morfologie fetala - Trimestrul I
- Ecografia de morfologie fetala - Trimestrul II
- Ecografia de morfologie fetala - Trimestrul III
- Boli oncologice si tratamente tumorale
- Bolile valvelor și ale vaselor inimii la copii
- Embolizare hemangiom / Tratament minim invaziv tumori benigne
- Fistula arterio-venoasa coronariana / Tratament legatura anormala artera si vena
- Fistula arterio-venoasa pulmonara / Tratament legatura anormala artera si vena pulmonara
- Stenoza pulmonara periferica / Tratament ingustare artera pulmonara
- Valvuloplastie pulmonara cu balon / Tratament ingustare valva pulmonara
- Boli ale aparatului genital masculin
- Boli cerebrale și ale coloanei vertebrale
- Anevrismul cerebral / Tratament durere de cap, paralizii preventie AVC
- Angioplastie carotidiana / Tratament boala carotidiana, atac ischemic vascular, AVC
- Discectomie cervicala cu DiscoGel / Tratament hernie de disc cervicala
- Malformatii arterio-venoase cerebrale / Tratament hemoragii cerebrale
- Nucleoplastie / Tratament hernie de disc
- Vertebroplastie / Tratament dureri coloana vertebrala fracturi de compresie
- Discectomie cu alcool
- Infiltrații transforaminale
- Boli ale articulațiilor



